ABOUT US
ABOUT US
Innovative Medical Repair Specialists
Oronix Inc. is proud to serve the North American medical market with a commitment to innovation, precision, and reliability. The medical industry demands exceptional technical expertise backed by significant investment, and Oronix meets that standard through advanced testing equipment operated by highly experienced engineers and technicians.
Every piece of faulty medical equipment is evaluated using a structured, systematic process. We have developed proprietary test procedures and custom jigs that significantly reduce repair turnaround times and overall costs, while maintaining an extensive inventory of genuine replacement parts to ensure efficiency and quality.
Our innovative, patented testing methods enable us to repair equipment that many other service providers cannot. In addition, Oronix offers extended warranties of up to two years on transducer repairs, providing our customers with confidence and peace of mind. With multiple patents to our name, Oronix delivers validated solutions that identify and eliminate critical flaws, ensuring long-term transducer performance and reliability.

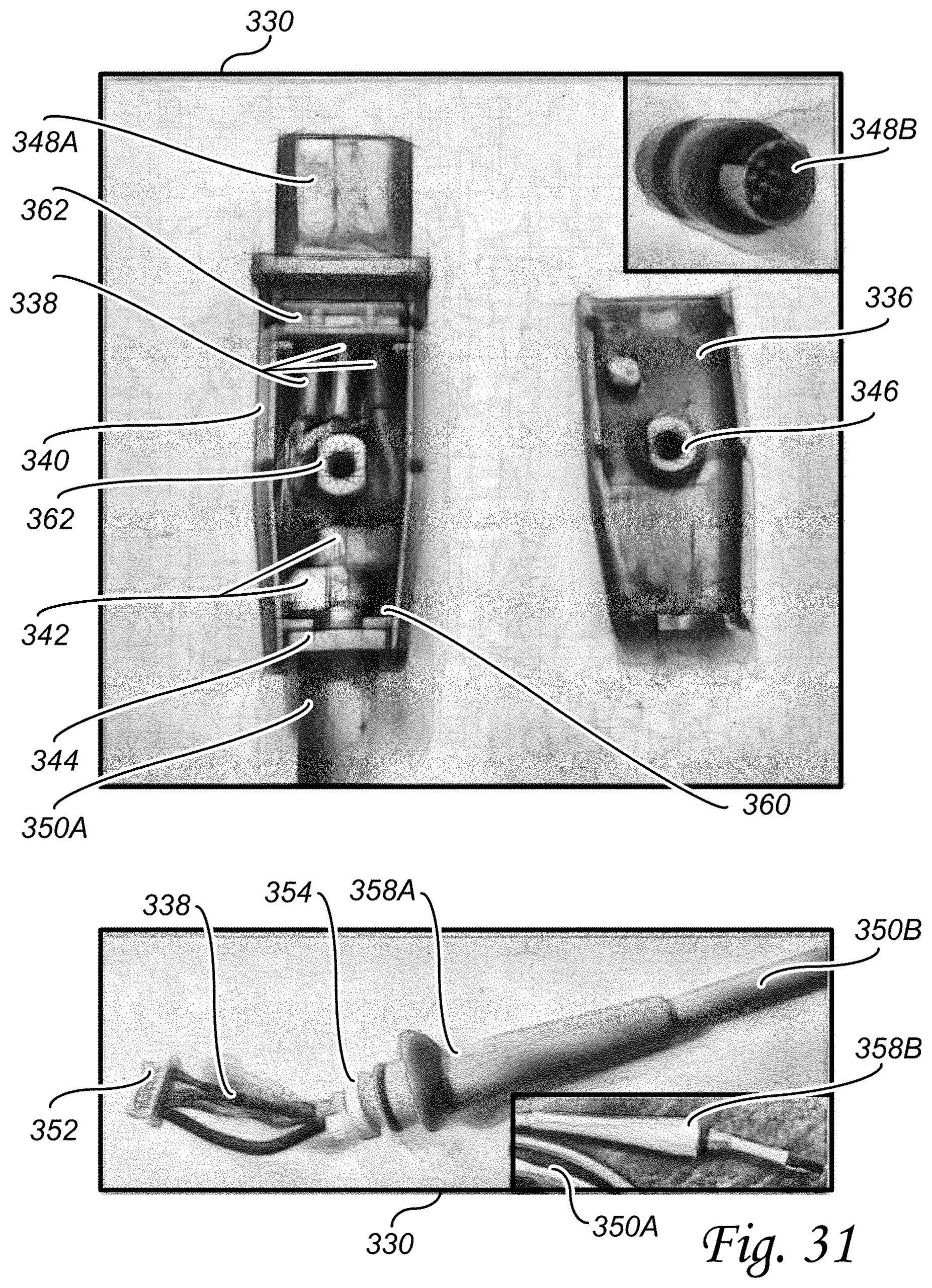
The present invention relates to an improved fetal heart rate transducer that overcomes shortcomings of prior transducers in several ways including utilizing an ultra slow-cure epoxy in combination with a piezo-electric disc bonding method to improve ultrasound transmission and reception characteristics, utilizing a slow-cure epoxy in combination with a frontend PCB bonding method to improve transducer reliability, utilizing a metal insert design in combination with molding-in techniques versus press fitting to improve the mechanical stability of the transducer, and utilizing a failsafe LVDS circuit to improve cable error detection and thus improve fetal monitor system reliability. In addition, a method of use of the present invention includes quantitatively determining the integrity of each of the piezo-electric disc bonds with the plastic substrate as well as the integrity of the ultrasound field using various height water phantoms that simulate the human body.
Fail-safe circuit for a low voltage differential signaling receiver
The present invention relates to differential receivers, and more particularly to a fail-safe circuit for low-voltage differential signaling (LVDS) receivers having single differential input disconnect detection with a latchable control signal interrupt capability. In operation, the receiver output is applied to a Vout output as long as the control signal is in a normal operating state, and on the first occurrence of a fault condition trigger is applied to the input of a latch, the latch latches applying a fault state to the control signal which causes the Vout output to follow the control signal blocking the receiver output until the latch is reset after the fault has been corrected.
Ultrasound beam quality test apparatus and methods
The present invention relates to an ultrasound beam quality test apparatus and methods of use. In this regard, fetal heart rate (FHR) transducer is placed for test and interconnected with fetal monitors. Phantoms of different heights can be placed on the FHR transducer. A computer system includes a beam control circuit. A plurality of hydrophone piezo-electric crystal (PZT) discs are placed on top of the phantom and interconnected with the beam control circuit. The computer system analyzes the ultrasound beam quality, of the FHR transducer, as it passes through the phantom. The beam control circuit can also control the oscillating motion of a metal plate to simulate a fetal heartbeat by way of a linear actuator. The FHR transducer registered heartbeat, by way of the fetal monitor, is then compared to the simulated fetal heartbeat to determine if the FHR transducer is working correctly.
The present invention relates to an improved tocodynamometer transducer that exhibits increased mechanical stability, dynamic range, accuracy, and reliability. Improvement components include top and bottom enclosures, plunger, ferrite core, LVDT transformer, transformer housing, flat spring, and other components. The flat spring includes four spring ribs that are symmetrical, curvilinear in shape, identical in path length, separated by an air gap, and equally spaced between the outer ring, inner ring, and the spring ribs that are adjacent. The spring constant is identical along two or more axes improving the accuracy of the improved tocodynamometer transducer. The ferrite core travel length is increased and mechanically constrained to remain between the LVDT transforming winding increasing the dynamic range and the linearity of the voltage output of the improved tocodynamometer transducer.
- US 63/728472 Pending Application (Not Available for Review)
The present invention relates to corrective solution for differential signaling (CAN & LVDS) to make it compliant with existing industry standard and eliminate spuriously false/phantom fetal heart rate readings in clinical Environment. Oronix is a leading solutions provider for medical and industrial enterprises. Our North American presence is a major factor in achieving short turn-around time, reliability and cost efficiency. The Oronix client gets timely service, quality products and professional customer support.
Oronix is a leading solutions provider for medical and industrial enterprises. Our North American presence is a major factor in achieving short turn-around time, reliability and cost efficiency. The Oronix client gets timely service, quality products and professional customer support.
Our Commitment
Turnaround time of 48 to 96 hours for cable repairs

Repairs by certified technicians

6 months warranty on all repairs (Extended 2 Years Warranty available)

Low cost of repairs; significant savings.
Long-Term Technical Solutions
Our staff works with your organization as a team finding long-term protocols, and this often uncovers causes external to the affected components. Weaknesses are often discovered in design and workmanship that lead to repeated failures, excessive downtime and repair/replacement costs. Oronix has the capability to fully evaluate a device and prepare accurate protocol for routine maintenance as well as provide technical training to on-site technicians, enabling the biomedical or clinical engineering departments to maintain cost effective operations.
